Regulation (EU) 2016/425
Protective clothing, protective gloves and respiratory masks all belong to the group of personal protective equipment (PPE) that have to be placed on the European market according to the regulations laid down in the Regulation (EU) 2016/425.
Regulation (EU) 2016/425 determines the fundamental safety requirements with which products have to comply and the procedures that have to be completed before placing a PPE on the internal market.
In this way, Regulation (EU) 2016/425 facilitates the free movement of PPE in the internal market with due respect of the minimal requirements regarding the consumer's safety and security (the so-called fundamental requirements). The actual technical elaboration of the fundamental requirements is based on harmonized European standards, which confer an irrefutable presumption of conformity with the legislation.
The producer is obliged to affix the CE-mark on his equipment as a visible indication of conformity with the fundamental requirements.
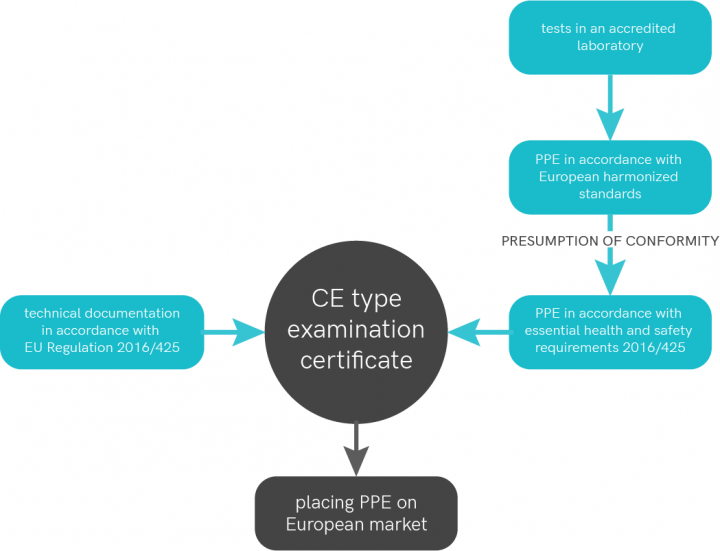
Regulation (EU) 2016/425 appeals to notified bodies for a number of tasks. This is especially the case for the CE-type examination of PPE belonging to categories II and III and for the monitoring of PPE of category III.
Since 1994, Centexbel is recognized by the FPS Employment, Labour and Social Dialogue as a notified body (European notification number 0493) for protective clothing and gloves. Centexbel is also the first Belgian Notified Body (# 0493) to be recognized to apply the new Regulation (EU) 2016/425 that harmonizes the legislation of the EU member states with respect to Personal Protective Equipment.
Categories
PPE are divided into 3 categories according to the gravity of the risk against which they protect. The category determines the procedure you have to complete before placing a PPE on the market.
Category I - PPE protecting against minor risks:
In this instance, it suffices to draw up a technical file. The manufacturer may then affix the CE-mark as a sign of conformity, without intervention of a third party.
Category II - PPE protecting against moderate risks:
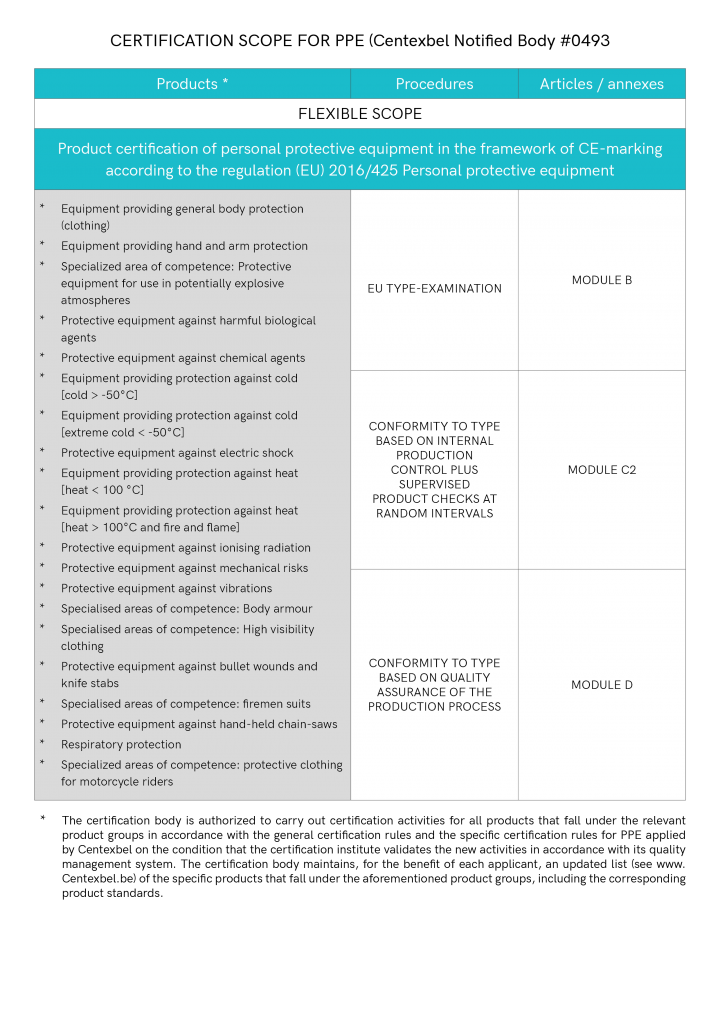
In addition to the technical file, a CE type examination has to be carried out by a notified body. The notification number of the notified body has to be mentioned in the information to the user. Only when the notified body has issued a type examination certificate, you are allowed to affix the CE-mark.
Category III - PPE protecting against mortal injuries or irreversible harm:
In addition to the technical file, and a CE type examination, the notified body has to carry out an annual quality monitoring. You may choose between two possibilities: either, the notified body takes samples from your production, which are then submitted to tests, or the notified body audits your quality system. Only when you are in possession of a CE type examination certificate and a positive report of the monitoring, you may affix the CE-mark. The CE-mark has to mention the notification number of the notified body performing the monitoring.
Declaration of conformity
The EC type examination certificate holder also produces a 'declaration of conformity' – a document including details of the company, information on the product, a list of the European Directives and the standards the product complies with, and a legally-binding signature on behalf of the organisation. The certificate holder has the responsibility to ensure that all supplied products are consistent with the type-approved model and continue to meet all the essential requirements of the PPE EU Regulation 2016/425.
Updated list
Centexbel on Nando
Centexbel is the first Belgian Notified Body (# 0493) to be recognized to apply the new Regulation (EU) 2016/425 that harmonizes the legislation of the EU member states with respect to Personal Protective Equipment, meaning that all PPE articles certified by Centexbel can immediately be freely marketed in all countries belonging to the European Economic Area (EEA).
Notification is an act whereby a Member State informs the Commission and the other Member States that a body, which fulfils the relevant requirements, has been designated to carry out conformity assessment according to a directive. Notification of Notified Bodies and their withdrawal are the responsibility of the notifying Member State.
Documents
Renewal CE Type-examination
The standard validity period for certificates is a maximum of 5 years from the date of original issue or date of re-issue. Any amendment, modification, revision, extension etc. of a certificate shall not change the original expiry date.
The expiry date is stated on each certificate.
Changes to any of the referenced standards during the 5 year period of the certificate will not affect the validity of the certificate, unless the presumption of conformity of a standard is withdrawn for safety concerns.
Certificates will not be renewed automatically.
If any company wishes to renew their certificate(s), a written application is required to cover the following:
- Confirmation of the current company name and address
- Confirmation of current production address(es)
- Confirmation that there have been no changes to the product, including sub-components/sub-assemblies
- Copies of current product drawings and photographs, product marking and information supplied by the manufacturer
- The data resulting from the control and test facilities that have been used to check compliance of the PPE with the harmonised standards and / or other technical specifications
- For category 3 products information on Module C2 status
The manufacturer is free to submit any additional documents to support the application for renewal, e.g. independent product certifications, independent quality system certifications, etc. The submitted documents will be reviewed against the requirements of the latest version of the PPE Directive within two months after receipt of all the required information and data, and if the notified body is satisfied that the product has not changed and remains in compliance with all requirements, certification will be re-issued, retaining the same certificate number, to be valid for an additional maximum of 5 years.
Where deficiencies are identified, the company will be requested to address these before certification is reissued.
If the notified body has any doubts about the current product being the same as that certified, they will be free to ask for more information, detailed drawings, photographs etc. plus if thought necessary, a sample of the model that is being questioned.
If the reference specifications / standards have been revised and published in the Official Journal, the notified body will review the changes against the existing data, and any requirements not satisfactorily addressed will be covered by independent product testing before certification is re- issued. Where a certification is not based on a harmonised standard the technical specification shall be reviewed against the PPE Directive to take into account evolution in associated or applicable standards.
The earliest application can be made 12 months before the expiry of the certificate and to ensure continuity of the certificate the application for renewal shall be made at least 6 months before the expiry date.
Where the referenced standard(s) have been superseded / amended and published in the Official Journal of the European Union within 12 months before the expiry date of the certificate, the validity of a certificate may be extended by a maximum of 12 months to give the manufacturer sufficient time to comply with the revised / amended standard(s).